Accuracy, Compliance, and Confidence in Every Trial
Clinical Trial Documentation
Turning complex information into clarity, compliance, and confidence.

Simplify Complexity
What we do
Clinical trial documentation is a critical part of the research process, providing detailed information about study design, methodology, and regulatory compliance. These documents ensure that trials are conducted ethically and scientifically, while serving as a permanent record for sponsors, regulatory authorities, and stakeholders. Accurate clinical documentation safeguards participant safety, supports regulatory adherence, and enables meaningful analysis and reporting.
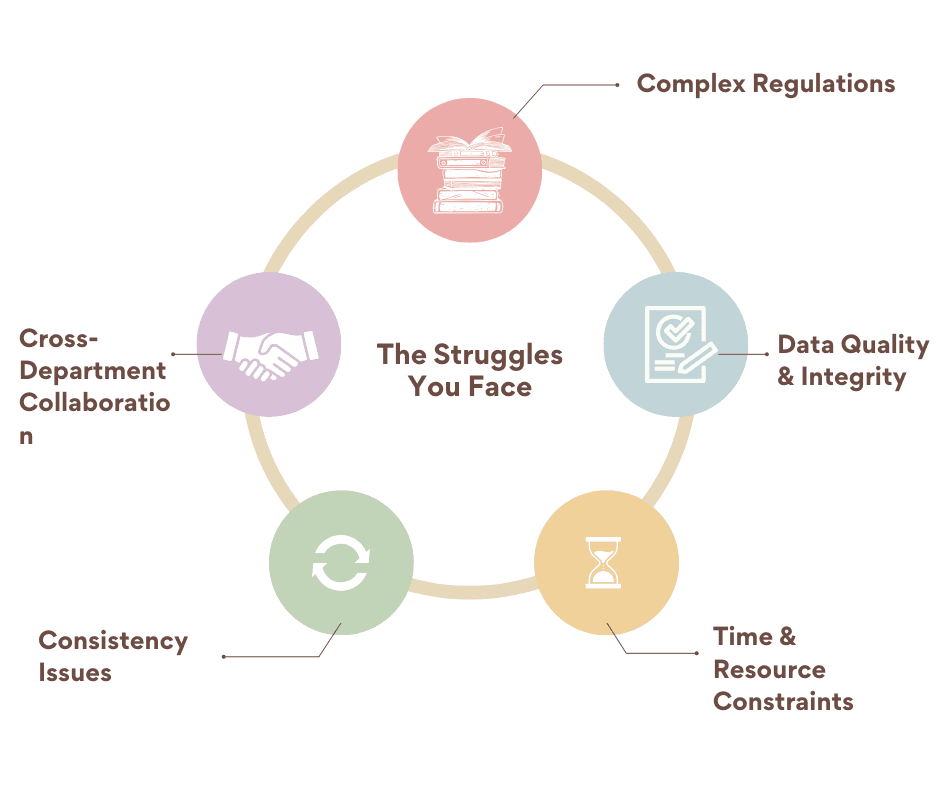
The Struggles You Face
Creating reliable clinical trial documents is complex and demanding. Teams often struggle with strict regulations, maintaining consistency, ensuring data quality, and coordinating across departments. Limited resources, tight timelines, and software limitations can further complicate documentation, while inconsistent quality or resistance to change can put compliance and trial integrity at risk.
Solutions
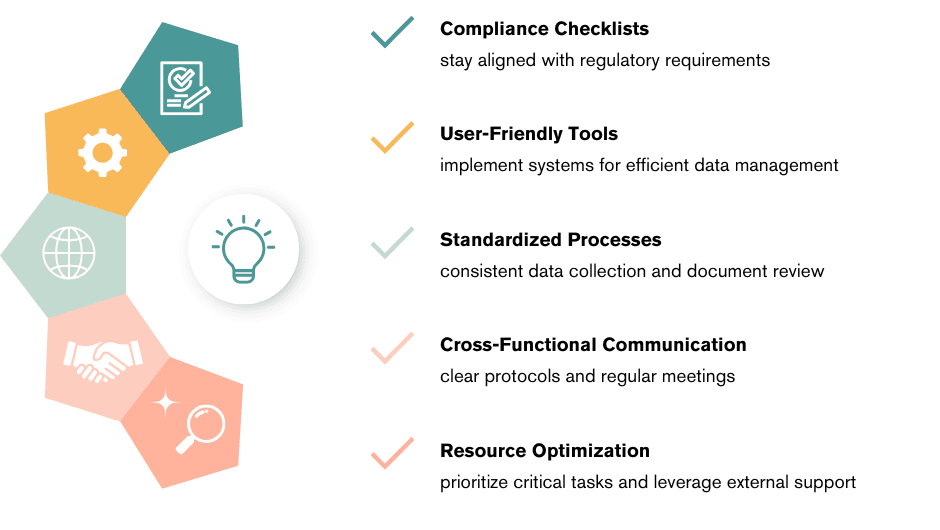
How We Make It Easier
We remove the stress from documentation by:
Why It Matters
Clinical trial documentation ensures safety, compliance, and reliability in every study. Clear, accurate, and standardized documentation provides a foundation for analysis, facilitates stakeholder communication, and contributes to medical knowledge.
Have a Project in mind?
We can help you bring your ideas to life. Let’s talk about what we can build and raise together.

More than writers we’re your partners in growth.
Connecting with us means having a reliable ally who ensures your documentation is clear, compliant, and impactful. Our goal is to lighten your load, empower your team, and contribute to the long-term success of your business.
