
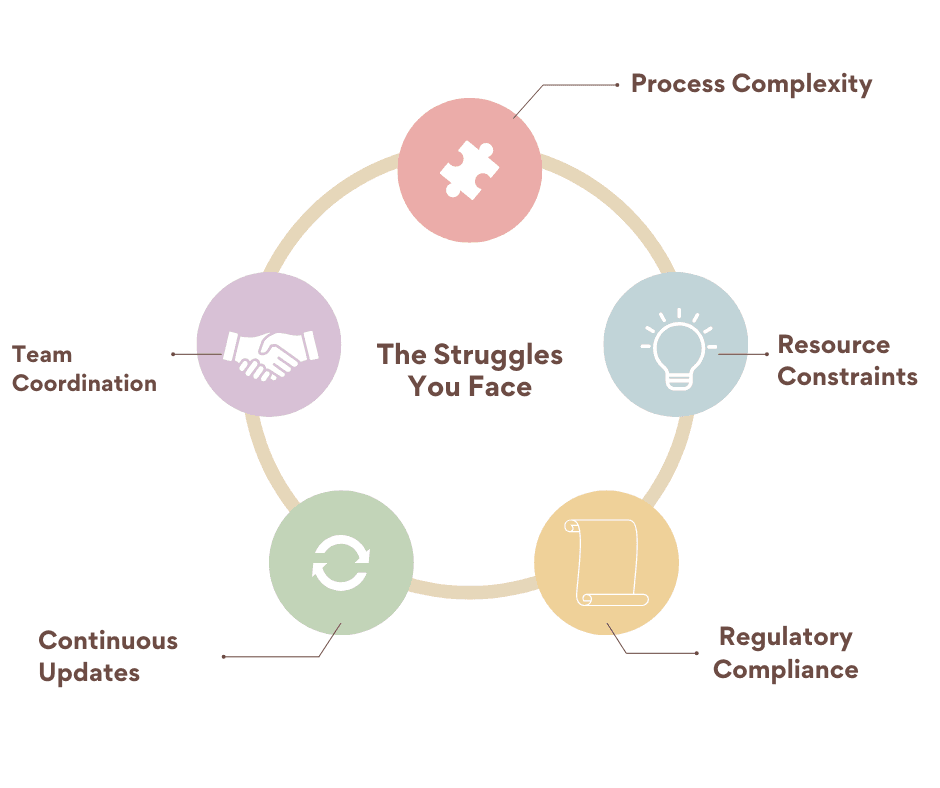
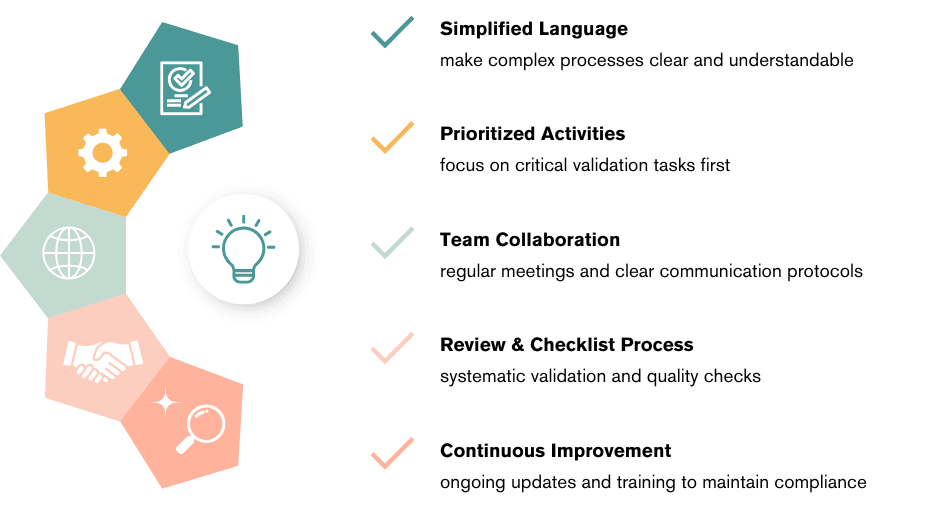

More than writers we’re your partners in growth.
Connecting with us means having a reliable ally who ensures your documentation is clear, compliant, and impactful. Our goal is to lighten your load, empower your team, and contribute to the long-term success of your business.
